An improved catalytic cracking of n-hexane via methanol coupling reaction over HZSM-5 zeolite catalysts
The coupling transformation of n-hexane and methanol over HZSM-5 has been investigated with a pulse-reaction system. In the temperature range of 400–500℃, kinetic data was collected and reaction order was determined. Compared with the pure n-hexane cracking, the increased rate constant and the lowered apparent activation energy clearly demonstrate an improvement of n-hexane activation using methanol as co-reactant and an increased contribution of faster bimolecular mechanism to the n-hexane transformation due to methanol introduction. Similarly, the results of coupling transformation performed over HZSM-5 with different Al content further confirm the transition between reaction mechanisms of n-hexane on account of the introduction of methanol. Moreover, the further investigation suggests that the enhancement of n-hexane activation and the change of reaction mechanism are attributed to the presence of intermediate species evolved from methanol. Thus, a proposed reaction pathway of n-hexane activation with methanol as co-reactant was put forward.
KEY WORDS: coupling transformation; mechanism; pulse-reaction; activation energy; Si/Al ratio.
1. Introduction
Hydrocarbon cracking is one of the most important processes for light olefins production in petrochemical industry. The disadvantage of this route is its high endothermicity, which makes it a very energy con-suming process. Some researchers have studied on catalyst development for high-efficient transformation and less energy cost, while some efforts are also put on alternative way, such as, introducing some exothermic conversion processes for energy supply into the endothermic hydrocarbon cracking. Considering the energy balance and target products, exothermic MTO/MTG process is a good option for this coupling system.
Lücke and co-workers investigated the coupling transformation of some hydrocarbons with methanol participation. A high olefins yield up to 1000 g kg)1h)1 in the temperature range of 600–700 ℃ in a nearly thermo-neutral condition was obtained, and the deactivation behavior of different modification HZSM-5 catalyst was also discussed. Gao and co-workers investigated the coupled conversion of methanol and light hydrocarbons over Ga/HZSM-5 catalyst at moderate temperature (<550 ℃), and studied the effect of reaction conditions on the yield of aromatics and lower alkenes. Shabalina and co-workers also worked in this field of methanol coupled conversion of propane and butane on MFI zeolite, and emphasized on the modification effect of alkaline-earth metals in the for-mation of light olefins.
In these studies discussed above, besides the consideration of energy supply, most of the study efforts were put on modifying reaction condition and zeolite catalyst for higher light olefins yield. However, for the conversion of methanol and the catalytic cracking of n-hexane, they are such reactions catalyzed by acid zeolite catalyst, although both reactions are quite different. When two reactions, thermally coupled each other, occur simultaneously, for the reactant and co-reactant, the feed of hydrocarbon and methanol, their transformations may not be independent completely. The chemical mechanism of the coupling transformation of n-hexane and methanol, especially the effect from methanol participation on the activation and conversion of hydrocarbon, is still obscure and merit further deep investigation.
In the present study, the transformation of n-hexane with and without methanol as co-feed was performed over HZSM-5 in a pulse reactor under the same reaction condition. The initial conversion rates of n-hexane at different temperature were tested, from which the rate constants of two reactions were deter-mined, then the apparent activation energies of
n-hexane in both reactions were calculated. The coupling conversion was also carried out over HZSM-5 with different Al content. By comparing the n-hexane alone conversion with methanol coupling n-hexane conversion, the change in activation energy of n-hexane and the effect of aluminum content on n-hexane conversion were discussed. Additionally, the effect of the species from methanol on n-hexane conversion was also investigated.
2. Experimental section
2.1. Catalyst preparation
Samples of HZSM-5 (Si/Al 13, 19, 25 and 70) were prepared with ion-exchanged method by exchanging NaZSM-5 (obtained from FuShun Catalyst Plant) with 0.5 M NH4NO3 solution at 80 ℃ for 4 h, and the operation was repeated 4 times, at last the ammonium sample was calcined in air at 550 ℃ for 4 h. Table 1 lists the physicochemical characteristics of these HZSM-5 samples.
2.2. Catalytic test
A pulse reaction system was used for all conversions. The catalyst (60–80 mesh) of 4.7–20 mg was loaded in the quartz reactor of 3 mm i.d. And quartz sands (60–80 mesh) were filled in the upper part of reactor to get plug flow state of mixture feed. A fresh catalyst was used on each run, and prior to use, the catalyst was pretreated at 550℃ for 1 h in a flow of N2.
The stream with certain amount of n-hexane for pulse reaction was generated by passing the carrier gas (He, >99.996%) of an appropriate flow rate through a saturator containing n-hexane at proper temperature. This stream was then mixed with the methanol stream with desired pressure generated in the same way, then the mixed stream was introduced into the reactor by the flow of helium. All products were separated and identified on-line by VARIAN CP-3800 gas chromatography equipped with a capillary column of PONA (100 m 0.25 mm) and a FID detector. Product analysis was reported by the DHA software. For comparison, the transformation of n-hexane alone with the same carbon atoms as co-reactant was carried out under the same condition.
The conversion of n-hexane and methanol was calculated based on the GC analysis using the following equation (where conversion of reactant and concentration of reactant in feed are expressed on molar carbon atom basis):

Transformation was performed with different contact time, which allowed extrapolation of conversion of n-hexane to zero contact time. As a result, the initial conversion rates of n-hexane were estimated from the tangent at zero contact time in the plot of the conversion versus contact time of n-hexane. The concentration of methanol in feed was fixed at 10% (C%) in most coupling experiments to limit the extent of methanol interconversion reactions.
3. Results and discussion
Varying the flow rate of carry gas with fixed contact time has no influence on the coupling reaction under the work conditions, suggesting no external limitation from diffusion control in the pulse reactor used. Blank test shows that the thermal transformation of n-hexane with and without methanol under the operating conditions is negligible.
Coupling transformation of n-hexane and methanol at different temperature
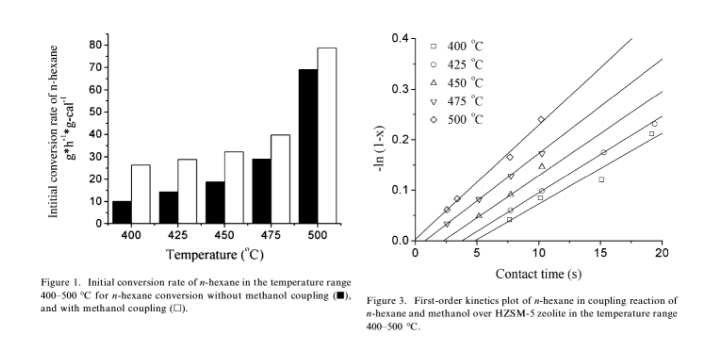
The experiments were carried out in the temperature range of 400–500 LC over HZSM-5 zeolite (Si/Al=19) with different contact time. The result in figure 1 shows the initial conversion rate of n-hexane in n-hexane alone cracking and coupling reaction experiments. Compared with the conversion of n-hexane alone, a clear increase of initial conversion rate of n-hexane is observed in coupling reaction, indicating a conversion enhancement of n-hexane by employing a coupling system with methanol as co-feed. It is also very interesting to find that the increase in conversion rate is more pronounced at low temperature than at high temperature compared with the uncoupled n-hexane cracking.
It is known that n-hexane cracking follows a first order kinetic rate law when the conversion of n-hexane is below 30% and the curve of )ln (1)x), in which x is the conversion of n-hexane, is expected to be linear as a function of the contact time. For n-hexane cracking over HZSM-5, the value of )ln (1)x) as a function of contact time plotted in figure 2 shows that in the studied temperature range, the conversion of n-hexane is a first-order reaction and the value of apparent rate constant can be calculated from equation: where k is the apparent rate constant of n-hexane con-version, s is the contact time of n-hexane (s).
While in the plot of methanol coupled n-hexane cracking, even the conversion has been improved in the whole temperature range, it can be still observed that the curve of )ln (1)x) is liner as the function of con-tact time as shown in figure 3. The apparent rate constants of n-hexane conversion listed in table 2 show that the first-order reaction rate constant k increases with reaction temperature, while in the whole temper-ature range, k from coupled reaction is always higher than that from the reaction of n-hexane without methanol introduction.
4. Conculsions
The coupling transformations of n-hexane and methanol over HZSM-5 zeolite catalysts, as well as the conversion of the hexane alone under the same conditions, have been investigated with a pulse reaction system. Comparing with the conversion of n-hexane alone, the increased rate constant and lowered apparent activation energy of n-hexane clearly show an improvement of n-hexane activation using methanol as co-reactant, and the increase of n-hexane conversion can be favored by the highly acid sites density and lower reaction temperature, which may be attributed to the presence of inter-
mediate species evolved from methanol. Therefore, it can be allowed to propose a reaction pathway, where the initiation step of n-hexane is dominated by faster bimolecular hydride transfer between the species from methanol and n-hexane molecule instead of the direct monomolecular protonation of n-hexane on Bronsted acid site of zeolite. As a result, the bimolecular progress will become a prevailing pathway in the coupling transformation of n-hexane and methanol.
Ceramic Carrier Materials in Heterogeneous Catalysis
Catalyst Carriers for the Chemicals Industry
Ceramic catalyst carriers form an important group of commonly used carrier materials in heterogeneous catalysis. They are primarily used in selective oxidation processes.

In heterogeneous catalysis bulk material catalysts are used to convert gaseous or liquid reactants. On an industrial scale fixed bed reactors are generally used for these types of reactions. The actual catalyst – i.e. the active catalytic substance – may be used alone or on a carrier. Carriers are used in situations where high demands are placed on the mechanical strength of the catalyst, the active catalytic substance must be present in a thin layer or there is a need to conserve valuable catalyst substances. A variety of materials are used to create catalyst carriers.
“Many intermediate and end products in the chemicals industry can only be produced with the help of catalysts.”
Ceramic catalyst carriers are an important group of carrier materials in heterogeneous catalysis and meet property requirements such as:
Chemical inertness
Mechanical strength and stability
Low surface profile
Bulk material uniformity
In this work zeolites HY, HZSM-5 and mixes of zeolites with γ-Al2O3 in different ratios were taken as carriers for 0.8 wt% Pd catalysts. Physico-chemical characteristics of the catalysts were determined by methods of Brunauer–Emmett–Teller (BET)–N2 adsorption, x-ray diffraction (XRD), scanning electron microscopy (SEM), energy dispersive x-ray spectroscopy (EDS), transmission electron microscopy (TEM), temperature-programmed reduction (TPR), hydrogen pulse chemisorption (HPC) and NH3 adsorption–desorption. The activity of catalysts was studied at 225–450 °C, at 0.1 and 0.7 MPa with molar ratio of H2:n-C6H14 = 5.92 and n-hexane concentration 9.2 mol%. Mixing of γ-Al2O3 with zeolite made acidity of catalyst weaken and led to a decrease of Pd cluster size, to an increase of Pd dispersity and a reduction of the extent of Pd in the case of catalyst Pd/HY; but for the catalyst Pd/HZSM-5 such mixing led to the reverse effect. That is why the increase of activity in the first case and the decrease of activity in the second case have been observed. It has been found that the optimal ratio of mixed carrier is γ-Al2O3:HY = 2.5:1 and the optimal calcined temperature of NH4ZSM-5 to obtain HZSM-5 is 500–550 °C. An increase of reaction pressure from 0.1 to 0.7 MPa remarkably increased the activity, selectivity and stability of Pd-based catalysts.