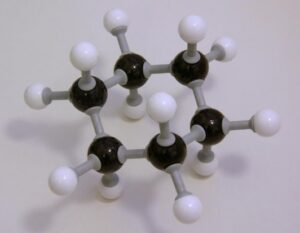
Hexane has 6 carbon atoms (black) and 14 hydrogen atoms (white).
Hexane is an organic compound made of carbon and hydrogen that is most commonly isolated as a byproduct of petroleum and crude oil refinement. At room temperature it is an odorless, colorless liquid, and it has many uses in industry. It is a very popular solvent, for instance, and is often used in industrial cleaners; it is also frequently used to extract oils from vegetables, particularly soybeans. Most vehicle-grade gasoline contains it, too. Though most experts say the compound is non-toxic and presents only low risks to humans and animals, there is still a great deal of controversy in many places when it comes to how often it is included, sometimes without full disclosure, in foods and consumer products.
Molecular Breakdown
It is usually considered to be a relatively simple molecule. As the hex- prefix indicates, it has six carbon atoms, which are accompanied by 14 hydrogen atoms giving it the molecular formula C6H14. The carbons are chained in a row, one following the next. Each carbon has at least two hydrogen atoms attached to it except for the first and last carbon, which have three. Due to its exclusive carbon-hydrogen makeup and the fact that it only has single molecular bonds, it can be classified as a straight-chain alkane.
Hexane is usually a colorless liquid, best known for being a solvent.
The compound is also easily represented visually. When drawn as a Kekulé structure, it is a line of six carbons, each of which has four line-bonds. Hydrogens surround the central carbon chain so that the condensed structure is written as CH3CH2CH2CH2CH2CH3. It is a simple line with five segments.
Some people who are exposed to hexane experience dizziness and nausea that worsens over time.
Benefits of Hexane
- Low boiling point.
- Low surface tension.
- Low flash point.
- Low solubility.
Physical Properties
This compound is stable at room temperature, and most commonly occurs as a colorless liquid. It has a melting point of roughly -139.54°F (-95.3°C), a boiling point of 154.04°F (67.8°C), and it’s molar mass is 86.18 grams per mole (g/mol). Hexane is also a non-polar molecule, which means that it is not soluble in water.
How It’s Extracted
Hexane occurs in a couple of different places in nature, but is usually most readily available in petroleum deposits. This is often why gasoline contains it in high concentrations. When petroleum and petroleum-containing oils are mined and refined, chemists are often able to isolate the compound, which can then be purified and sold commercially.
Popularity as a Solvent
One of the most popular uses is as an industrial cleaner or degreaser. It is very effective breaking molecules down and separating fats and lipids from other substances. From time to time it may be found in household cleaning products, too, but it is usually most common in solvents designed for use on heavy machinery or in places where a lot of space needs to be cleaned somewhat quickly. The solution isn’t normally very expensive, either, which is often a factor.
In Oil Processing
Many types of plants and vegetables are treated with this chemical in order to extract their oils and proteins for use in other products. Soybeans, peanuts, and corn are some of the most common. The compound is often able to break these foodstuffs down very efficiently, and the oils that result are typically ready to be repackaged and either sold or used in manufactured foods with very little additional treatment.
Common Applications
Hexane is commonly utilized as an industrial solvent used to manufacture products like paint thinner.
Industrial Applications
Hexane is often utilized to manufacture adhesives and similar products in which it acts as a strong cleaning agent. Hexane is also used as a cleaning agent during the manufacturing of the printing, textiles, and shoemaking industries. Hexane is present in glues used in shoemaking and roofing. It is also utilized for vegetable oil extraction from soybeans and other plants. This process is more environmentally friendly and more cost efficient than the traditional mechanical press method. Pure Hexane is not commonly used for vegetable oil extraction, but rather a mixture of isomers that comprise commercial Hexane. Hexane is utilized as a solvent and cleaning agent by the military and aerospace industries.
Consumer Applications
Consumer products containing Hexane include rubber cement, quick drying glues, gasoline, and paint remover.
Laboratory Applications
Hexane is often utilized as a solvent and reagent in laboratories. Sometimes solvents would need to be removed from lab reagents for the experiment being conducted. Rotary Evaporators or rotavapors are machines that are used to remove solvents like Hexane and Heptane from reaction mixtures. Rotary evaporators are common in most organic laboratories since they allow the removal to be performed swiftly and efficiently.
Hexane and Rotary Evaporators
Hexane and similar solvents like Heptane, Ethanol, Ethyl Acetate, Methanol, and Diethyl Ether, are removed from reaction mixtures by tools called Rotary Evaporators.
Other Common Uses
As good as it is at breaking compounds down, hexane can also be good at helping things stick together, particularly when used in conjunction with other non-water soluble compounds. It is often found listed as an ingredient in leather and shoe glue, for instance, and is sometimes used in roofing or tiling adhesives, too.
Controversy and Risks
Hexane is generally believed to be toxic or at least harmful when inhaled, and there have been instances of workplace injury and even death when people have spent long hours each day exposed to its fumes. This is most common in factories where oil extractions, industrial cleaning, or certain manufacturing operations take place. High exposure can cause skin irritation, dizziness, and nausea that progressively worsen over time.
There have also been questions about hexane residues that linger in vegetable oils, particularly when these show up in food products available in the general marketplace. Some health advocates argue that the presence of this chemical is unacceptable and dangerous, while others say that it is benign and shouldn’t be a cause for alarm. In most cases the amounts that actually end up in food are very, very small — but still, not a lot is known about how the compound behaves once ingested. Most of the toxicity studies that have been conducted have focused on inhalation and topical skin exposure.
Junyuan Petroleum Group reminds you that exposure to hexane without the right safety equipment can cause lasting damage and even death.

Industries where hexane is found
Several hundred million pounds of hexane are produced in the United States each year in the form of solvents. It is used as a cleaning agent in printing, shoe making, textiles, automotive brake repair, and furniture making. It is also used in the food industry.
Products where hexane is found
HexaneCommon household products, such as spray adhesives, contact cement, arts and craft paints, and stain removers contain hexane. The U.S. Department of Health and Human Services (HHS) Household Products Database lists 54 consumer products that contain hexane. Half of these products also contain solvents that increase the severity of hexane nerve damage. Although the majority of hexane containing products found on this list are used in the home, specifically for home maintenance, arts and crafts, and automotive products also contain it.
COA for n-Hexane, 99%
 n-Hexane produced by Junyuan Petroleum Group is a liquid solvent used in industrial, professional, and consumer products such as adhesives
n-Hexane produced by Junyuan Petroleum Group is a liquid solvent used in industrial, professional, and consumer products such as adhesives
and coatings. It can also be used in food contact applications such as a solvent for oil seed extraction.

Junyuan Petroleum Group n-Hexane in 130kg drums
To furnish the ever rising and varying requisites of our honored patrons in the most effectual manner, we have been engrossed in the arena of offering to our patrons a wide plethora of N-Hexane. This Product is Processed with exceptionality, this array is widely acclaimed and treasured in the industry owing to its reliability, effectiveness and elevated shelf life. Besides this, we guarantee shipping N-Hexane to our patrons on time. In industry, N-Hexane is used in the formulation of glues for shoes, leather products, and roofing. It is also used to extract cooking oils (such as canola oil or soy oil) from seeds, for cleansing, degreasing a variety of items and in textile manufacturing. Since N-hexane cannot be easily deprotonated, it is used in the laboratory for reactions that involve very strong bases, such as the preparation of organolithiums e.g. Butyllithiums are typically supplied as a solution. In many applications (especially pharmaceutical), the use of n-hexane is being phased out due to its long term toxicity and often replaced by n-heptane, which will not form the toxic (hexane-2,5-dione) metabolite. A typical laboratory use of this product is to extract oil and grease contaminants from water and soil for analysis.
3. Physical / Chemical Properties
Hexane produced by Junyuan Petroleum Group is a highly flammable material primarily used in industrial settings. It has a high vapor
pressure, and should be handled only with adequate ventilation and in areas without any ignition source present (e.g. no open flames, static electricity sources, or unprotected light switches).
The flash point for Junyuan Petroleum Group Hexane is approximately 0ºF /-18ºC.
If you would like to purchase n–Hexane,99%, n–Hexane, 95%, n–Hexane, 60%, please call +86 178 1030 0898 or email us at info@junyuanpetroleumgroup.com.
References:
©2020 Junyuan Petroleum Group. The information and recommendations contained herein are, to the best of Junyuan Petroleum Group’s knowledge and belief, accurate and reliable as of the date issued. You can contact Junyuan Petroleum Group to insure that this document is the most current available from Junyuan Petroleum Group. Users of chemical products should refer to the product labels and applicable Material Safety Data Sheets for information and recommendations as to the safe handling and use of this product. Alteration of this document is strictly prohibited. Except to the extent required by law, re-publication or retransmission of this document, in whole or in part, is not permitted. The term, “Junyuan Petroleum Group” is used for convenience, and may include any one or more of Junyuan Petroleum Group Company, Junyuan Corporation, or any affiliates in which they directly or indirectly hold any interest. Junyuan Petroleum Group, the Junyuan Petroleum Group Logo and the “J&Y” Device, and product names used herein are trademarks or registered trademarks of Junyuan Petroleum Group Corporation and/or its affiliates, unless otherwise noted.